Batch management with UDI labelling for medical devices and medicines
UDI labels with all the relevant information
Batch management for products from the medical sector is regulated by the Medical Devices Act (MPG) of 1 January 1995. All companies that manufacture medical devices are obliged to label their products with a batch number (manufacturers, importers and retailers of medical devices). Under certain circumstances, batch number-based product recall actions can be initiated to protect consumers from harm.
This obligation applies in particular to all implantable products, in-vitro diagnostics and all products manufactured from animal substances.
On 26 May 2020, the transitional period for the entry into force of the ‘Medical Device Regulation’ (MDR) expired. The demands made by the EU in the MDR became obligatory for all medical sector companies and manufacturers. One main MDR requirement is the traceability of products with batch and lot numbers.
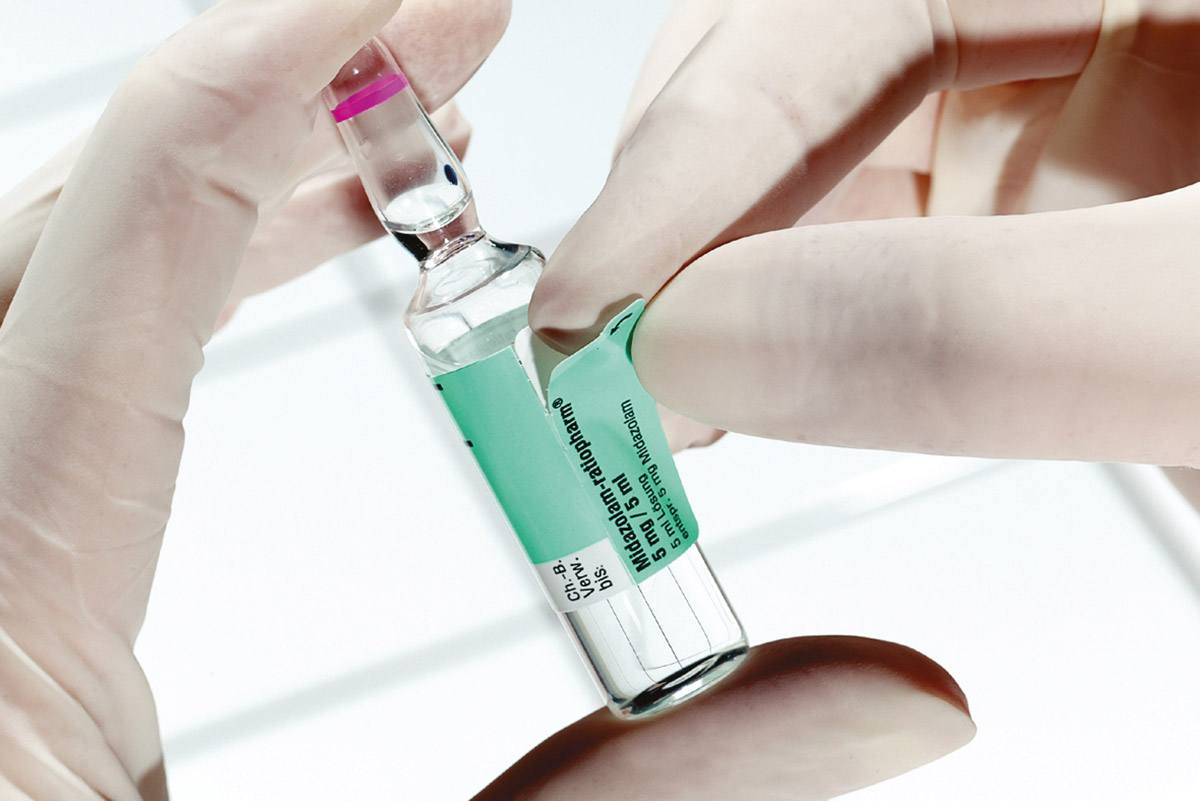
The usage scenario: identification of medical devices in the production process
In the pharmaceutical industry, the batch number contains information on shelf life, expiry date and product details of the medical devices.
The basis for efficient traceability is a standardised label that contains all the relevant information. In the medical sector, this standard is the ‘UDI’ (Unique Device Identification) or Product Identification Number.
Among other information, it contains the batch number of the product (in the medical sector, a batch number is known as a lot number). The UDI consists of two parts, the UDI-DI ("Device Identifier") for identifying the product and manufacturer and the UDI-PI ("Production Identifier") which marks the batch of a product. The data in the UDI marking form the basis for traceability and are available in a standard, digitally readable form.
All the necessary information, such as article number, GTIN (Global Trade Item Number), batch number and expiry date is encoded in the "Unique Device Identification" (UDI) in one barcode, usually in the form of a 2D barcode, e.g. GS1 data matrix codes or a GS1 128 barcode, with a plain text line for the consumer.
The UDI replaces the previous PZN information (Pharmacy Central Number) and best-before or expiration date.
The advantages: all the information on the label can be captured with a barcode scan, errors due to manual entries are avoided, and the capturing procedure is much faster. Labelling should be integrated as much as possible into the production process – this will avoid wrong allocations at the interfaces.
According to the Medical Devices Regulation (MDR), a large amount of data on medical devices must be stored in the European database for medical devices (EUDEMED). EUDEMED is designed to improve market surveillance and provide competent authorities with rapid access to product information.

The solution: laser-activated labels – no-contact printing in the packaging process
Labels offer several advantages because they always place the same demands on the printing system – and they can be applied at the same speed in the packaging line. Different folding box sizes can be labelled with just one type of label.
Laser-activated labels, are printed and dispensed inline in the packaging process. They are equipped with a sophisticated embellishment – a laser ‘inscribes’ them in black directly in the labelling system.
Plain text, graphics and/or codes – the print image is razor-sharp, even with the smallest font sizes. The print is smudge-proof and scratch-resistant.
The laser printing system requires no additional consumables and is not subject to wear and tear. This considerably reduces production downtime.
Labels on rolls for thermal transfer printers can also be printed and dispensed de-centrally near the production line(s). Many standard label formats are available for this purpose. Of course, we also produce individual label sizes that are adapted to your packaging size. HERMA also offers suitable labeling machines, for labels on rolls that are printed locally (near the production line(s)). The machines are then fully integrated into your packaging systems.

What’s special about laser-activated labels for this usage scenario?
- System solution: labels, printing system and labelling unit, all from one source
- Laser-activated labels: razor-sharp print image, ideal for data matrix codes
- Wear & tear-free, no additional consumables
- Highest level of process security
More interesting solutions for the pharmaceutical industry
Documentation labels in hospitals ensure that drugs, medicated plasters and dressings are correctly linked to patient records. They are also used on vaccine packages for use as documentation in patient records and vaccination cards. The labels are designed in such a way that parts or all of the label can be detached and affixed to another surface after the main label has been attached
Counterfeit protection also plays an essential role in the pharmaceutical industry. Here too, labels make a decisive contribution as sources of information for traceability labelling, as a security feature to protect against product piracy and provide proof of product authenticity and clear evidence if the seal has been broken (tamper-evident labels).
We are here for you – get in touch with us!
Individual solutions are the standard, especially in the medical sector. We would be pleased to develop the optimum solution for your usage scenario – together with you. From a wide range of label materials from our in-house production, we will select the right combination of label material and adhesive, perfectly tailored to your printing systems and requirements. Use the contactform to let us know what you need – we’ll get back to you!
Learn more about
Laser Labels: LAM labels for CO2 labelling systems
Patients’ labels and documentation labels
HERMA Tamper Evident labels
Counterfeit protection through serialisation in the pharmaceutical industry